Highly pathogenic avian influenza (HPAI) viruses pose a significant threat to animal and human health and have tremendous negative economic impacts. Generally, avian influenza type A viruses are categorized based on the severity of the disease in chickens into two categories, HPAI and low pathogenic avian influenza (LPAI).
Although the number of infected human cases is not very high, HPAI has had devastating economic consequences due to its effect on the poultry industry with nearly 100% mortality in chickens and approximately 60% reported mortality in human cases [1]. As an example, based on statistical analysis from multiple sources including the US Department of Agriculture (USDA), Animal and Plant Health Inspection Services (APHIS), the Livestock Marketing Information Center (LMIC), and the Egg Industry Center (EIC) at Iowa State University, the 2015 HPAI outbreak resulted in 15,693 fewer jobs, $981 million in lower value-added products, $2.6 billion in lower output of poultry production, and estimated reduction in total federal and state and local tax receipts by $248.6 million and $136.1 million, respectively.
Avian influenza or bird flu is known to be a highly contagious respiratory disease of birds caused by the RNA virus from the Orthomyxoviridae family. There are four types of influenza viruses in the Orthomyxoviridae family and these are classified as types: A, B, C, and D. Type A influenza virus is the most widespread and carries zoonotic (can be transmitted from animals to humans) capabilities that can infect different birds and mammalian species that nourish their young with milk, including humans. Type B and C influenza viruses infect humans and rarely other species, while type D infects fish. Type A has been divided into different subtypes based on the surface protein present in the virus. The proteins, hemagglutinin (HA) and neuraminidase (NA), themselves have many subtypes. HA has been known to have 18 subtypes and NA has 11 subtypes among which 16 HA and 9 NA subtypes are found in birds. There can be many combinations of the surface protein, producing various subtypes of avian influenza virus.
Essentially, interaction between wild birds and poultry is the primary factor that causes the transmission of HPAI and the evolution of LPAI into HPAI. Wet markets that typically house multiple bird species at high density, with new bird species from different geographical areas arriving daily, are places where avian influenza viruses can easily exchange their subtypes through the fecal-oral route due to poor biosecurity and low hygiene standards [2]. Backyard poultry, domestic ducks, and geese, which are generally reared in free-range settings, also directly and indirectly interact with wild birds which can add to the evolution of HPAI subtypes [3]. Further, bird bridge species like sparrows, pigeons, and corvids may also play a major role in the emergence of HPAI since they live near poultry and encounter other wild birds.
Studies show that wild birds are primary wild reservoirs (a host that directly or indirectly transmits a pathogen) for avian influenza viruses (AIV) [4]. Wild birds, especially those that are migratory, play a major role in transmitting HPAI H5N1 viruses since they can carry bird flu without showing symptoms and may spread the virus over wide areas [5]. Influenza viruses can cause severe illness and death in domestic poultry. These viruses can infect many birds, including free-living and captive caged birds, domestic ducks, chickens, turkeys, and other domestic poultry, including through wild bird transmission. Influenza A viruses also circulate in waterfowl [6].
Research has shown that a majority of migratory birds may transmit avian influenza viruses, including various waterfowl species such as ducks, geese, cranes, and gulls. Tests done on migratory waterfowl showed clinical signs like paralysis, head tilt, staggering gait, and injury in multiple organs; therefore, this virus can cause mortality in migratory birds [7]. The role of migratory birds is not limited to spreading HPAI infection: research has also shown that LPAI viruses in migratory birds may mutate within these birds or before getting into the poultry population, which may later mutate to deadly HPAI viruses [7].
One of the vectors that enables transmission of the HPAI H5N1 virus includes water in lakes and ponds where wild birds like waterfowl congregate during their migration [5]. Waterfowl congregation in a particular geographical region increases the probability of infecting domestic avian species since these birds are considered a natural reservoir for most of the AI virus subtypes like the H5N1 virus [7]. During winter, wild birds migrate due to cold weather and transfer the virus to other new areas. Cold weather also favors the virus’ survival [8]. AI viruses are associated with migratory water birds and must have adapted with their host behavior to accommodate the migration cycle. The virus can survive for weeks or months in cold water, and for several years in ice bodies. For example, AI viruses were isolated from ice in lakes in Siberia at a time when wild birds had already moved out of the region [9]. Infected birds also shed avian influenza virus through feces, saliva, and mucous [10]. Research has shown that considerable quantities of the virus are excreted with the feces and therefore end up contaminating lakes and ponds. The virus may remain in the water and be infective for up to four days at 22°C and over 30 days at 0°C [5].
Some birds like waterfowl undertake regular migration between the world’s hemispheres and could introduce HPAI H5N1. Further demonstrating the H5N1 virus’ transmissibility, an outbreak in Asia spread to Russia, the Middle East, Europe, and Africa and resulted in an increased focus on the role of wild birds. Another mode that can transport the HPAI H5N1 virus is vagrancy, sweeping up and transporting Old World birds, especially waterfowl, across the Atlantic to the New World through tropical storm systems during the Atlantic hurricane season. Although the method is rare, Old-World birds can be swept by a hurricane during hurricane season and transported worldwide. Legal and illegal importations of birds can also be a mode of transporting the HPAI H5N1 virus. Although a quality assurance (QA) system is in place for legal importation, which ensures birds are quarantined and tested, research has shown that tested birds are at times found with the virus thus indicating that importation can be a mode of transporting the virus, especially through illegal importation since a strong QA system is not always in place.
Regional Example: Past and Current History of Highly Pathogenic Avian Influenza in North America
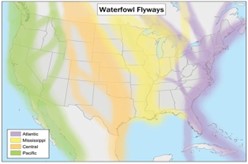
As a result of the coexistence of many avian influenza viruses (AIVs) among domestic and wild birds, a diverse viral gene pool has emerged since the first report of H5N1 HPAI in Asia in 1996. Multiple viral reassortants such as H5N2, H5N5, and H5N8 have developed from the Asian H5N1 HPAI. In the 18 months following May 2013, AIVs were detected in nearly twice as many birds across the world, both domestic and wild. In the fall of 2014, commercial poultry in Southern British Columbia, Canada, was found to have H5N2. Further testing revealed the presence of two HPAIs in wild birds in the United States, as well as mortality events associated with captive raptors. By January 2015, surveillance activities that focused on wild birds from hunter-killed animals indicated that all states in the Pacific Flyway sampled for HPAI had at least one positive finding. Multiple outbreaks had been reported in backyard and commercial poultry, along with additional detections in other flyways. Flyways are broadly defined as corridors where the migratory paths of many species of interest tend to unite.
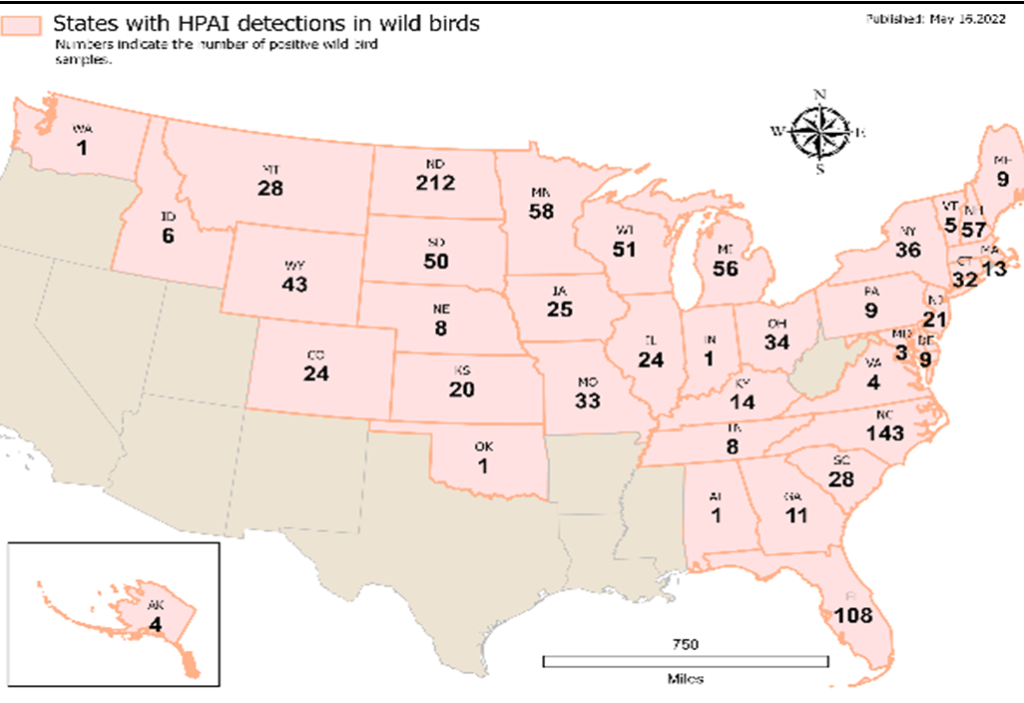
The first 2021/2022 detection of Eurasian strain (EA) highly pathogenic avian influenza HPAI H5N1 in North America occurred in December 2021 in Newfoundland and Labrador, Canada. Subsequently, HPAI EA H5 and EA H5N1 viruses have been confirmed in commercial poultry facilities, wild birds, backyard flocks, and wild mammals in both Canada and the United States. The Centers for Disease Control and Prevention (CDC) has been monitoring for illness among people exposed to H5N1 virus-infected birds since these outbreaks were detected in U.S. wild birds and poultry in late 2021 and into 2022. To date, H5N1 viruses have affected 46,430,000 U.S. commercial and backyard birds in 40 states with the latest instances including on Sept 15, 2022 in Obion County, Tennessee; 1,200 wild birds in 38 states [11]; and a human case in one state [10]. The CDC has tracked the health of more than 2,500 people with exposures to H5N1 virus-infected birds and found only one case to date in Colorado. Other people involved in the culling operation in Colorado have tested negative for H5 virus infection, but they are being retested out of an abundance of caution [10].
Migratory Waterfowl Flyways in the US
Waterfowl and waterbird migration in North America consists of north-south seasonal movements between breeding grounds and wintering areas. The four flyways in North America include the Atlantic, Mississippi, Central, and Pacific. Research shows that the Pacific Flyway is thought to be the most likely area of introduction for the HPAI viruses detected in Canada and the United States in December 2014.
The first detection of Asian HPAI H5 viruses in United States wild birds since 2016 was recently announced by the USDA Animal and Plant Health Inspection Services (APHIS). Human infection is reported to be rare with HPAI H5N1 but can usually occur after close contact with infected birds; therefore, people with close or continued unprotected contact with infected birds or contaminated environments may be at more significant risk of infection.Most cases cause minor sickness or no noticeable signs of disease. Unlike before, all confirmed LPAI H5 and H7 A1 subtypes are now reported to the World Organization of Animal Health (OIE) because of their potential to mutate into highly pathogenic strains as once detected in Lancaster County, Pennsylvania in 1983-84, which caused many birds to be depopulated [11].
Overall, the HPAI virus is a potential zoonotic disease in which wild birds play a vital role in transmission of the disease to poultry populations, causing economic losses in the poultry industry. Protecting our national poultry investments from bird flu helps protect farmers’ livelihoods. It is crucial that the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS), and Center for Diseases Control and Prevention (CDC) work closely with states and the poultry industry to prevent AI from establishing itself in the U.S. poultry population.
References
[1] Claas ECJ, Osterhaus ADME, van Beek R, De Jong JC, Rimmelzwaan GF, Senne DA, et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998; 351:472–7
[2] Cardona, C., Yee, K., & Carpenter, T. (2009). Are live bird markets reservoirs of avian influenza? Poultry Science, 88(4). https://www.sciencedirect.com/science/article/pii/S003257911939162X
[3] Takekawa, J. Y., Prosser, D. J., Newman, S. H., Muzaffar, S. bin, Hill, N. J., Yan, B., Xiao, X., Lei, F., Li, T., Schwarzbach, S. E., & Howell, J. A. (2010). Victims and vectors: Highly pathogenic avian influenza H5N1 and the ecology of wild birds. In Avian Biology Research (Vol. 3, Issue 2, pp. 51–73). https://doi.org/10.3184/175815510X12737339356701
[4] Bevins, S. N., Dusek, R. J., White, C. L., Gidlewski, T., Bodenstein, B., Mansfield, K. G., Debruyn, P., Kraege, D., Rowan, E., Gillin, C., Thomas, B., Chandler, S., Baroch, J., Schmit, B., Grady, M. J., Miller, R. S., Drew, M. L., Stopak, S., Zscheile, B., … Deliberto, T. J. (2016). Widespread detection of highly pathogenic H5 influenza viruses in wild birds from the Pacific Flyway of the United States. Scientific Reports, 6. https://doi.org/10.1038/srep28980
[5] Alexander, D. J. (2007). An overview of the epidemiology of avian influenza. Vaccine, 25(30 SPEC. ISS.), 5637–5644. https://doi.org/10.1016/j.vaccine.2006.10.051
[6] Tong, S., Li, Y., Rivailler, P., Conrardy, C., Alvarez Castillo, D. A., Chen, L. M., Recuenco, S., Ellison, J. A., Davis, C. T., York, I. A., Turmelle, A. S., Moran, D., Rogers, S., Shi, M., Tao, Y., Weil, M. R., Tang, K., Rowe, L. A., Sammons, S., … Donis, R. O. (2012). A distinct lineage of influenza A virus from bats. Proceedings of the National Academy of Sciences of the United States of America, 109(11), 4269–4274. https://doi.org/10.1073/pnas.1116200109
[7] Dhama, K., Mahendran, M., & Tomar, S. (2008). Pathogens Transmitted by Migratory Birds: Threat Perceptions to Poultry Health and Production. International Journal of Poultry Science, 7(6), 516–525.
[8] Ellis, T. M., Bousfield, R. B., Bissett, L. A., Dyrting, K. C., Luk, G. S. M., Tsim, S. T., Sturm-Ramirez, K., Webster, R. G., Guan, Y., & Peiris, J. S. M. (2004). Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathology, 33(5), 492–505. https://doi.org/10.1080/03079450400003601
[9] Zhang G, Shoham D, Gilichinsky D, Davydov S, Castello JD, Rogers SO. Evidence of influenza A virus RNA in Siberian lake ice. J. Virol. 2006;80:12229–12235.
[10] Center for Disease Control and Prevention (CDC). 2022. Current U.S. Bird Flu Situation in Humans
[11] US Department of Agriculture (USDA), Animal and Plant Health Inspection and Services (APHIS). 2022. Avian Influenza. https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/avian/avian-influenza

